SLIDE CULTURE TECHNIQUE
The slide culture technique is a laboratory method used for the identification of fungi, especially those that produce characteristic structures such as spores and hyphae. This technique allows fungi to grow in a controlled environment while preserving their natural morphology, which is crucial for accurate identification under a microscope based on the structure.
Fungal structures examined under the microscope include hyphae (filamentous structures that form the mycelium), which can be septate (divided by cross-walls) or aseptate (continuous without septa).
Reproductive structures such as spores are also key identifiers. These include asexual spores (e.g., conidia and sporangiospores) and sexual spores (e.g., ascospores, basidiospores). Specialized structures like conidiophores (which bear conidia), sporangia (sacs containing sporangiospores), and fruiting bodies (multicellular reproductive structures) provide additional diagnostic details.
Other structures of interest include chlamydospores (thick-walled survival spores), arthrospores (formed by hyphal fragmentation), and yeast cells (unicellular fungi reproducing by budding). Pseudohyphae (chains of yeast cells resembling true hyphae), along with rhizoids (root-like structures) and stolons (horizontal connecting hyphae), are also examined.
This technique often employs staining methods like lactophenol cotton blue, calcofluor white, or KOH mounts to enhance visibility and detail of these fungal structures, aiding accurate identification.
Procedure
This procedure adapted from Senanayake et al., 2024.
1. Equipments
Everything should be sterilized:
- Ose needle
- Petri dish (disposable)
- Slide and cover glass
- Spatula or scalpel
- V-shape glass or metal rod
- Water
- Pipette or Micropipette (sterilized the tip for micropipette)
- Forceps
- Ethanol in the glass jar
- Potato dextrose agar medium added by antibacterial and poured into the Petri dish
2. Processes
The slide culture preparation must be done in Biosafety Cabinet (BSC).
Step 1: Prepare all equipment in the BSC and put the forceps and spatula or scalpel into the ethanol in the jar.
Step 2: Put the V-shape glass rod to the Petri dish.
Step 3: Add the sterile water 500 uL to the Petri dish.
Step 4: Put the slide glass to the V-shape glass rod, please put it carefully and avoid too many movements for making the slide glass keep on the position. (Figure 1)
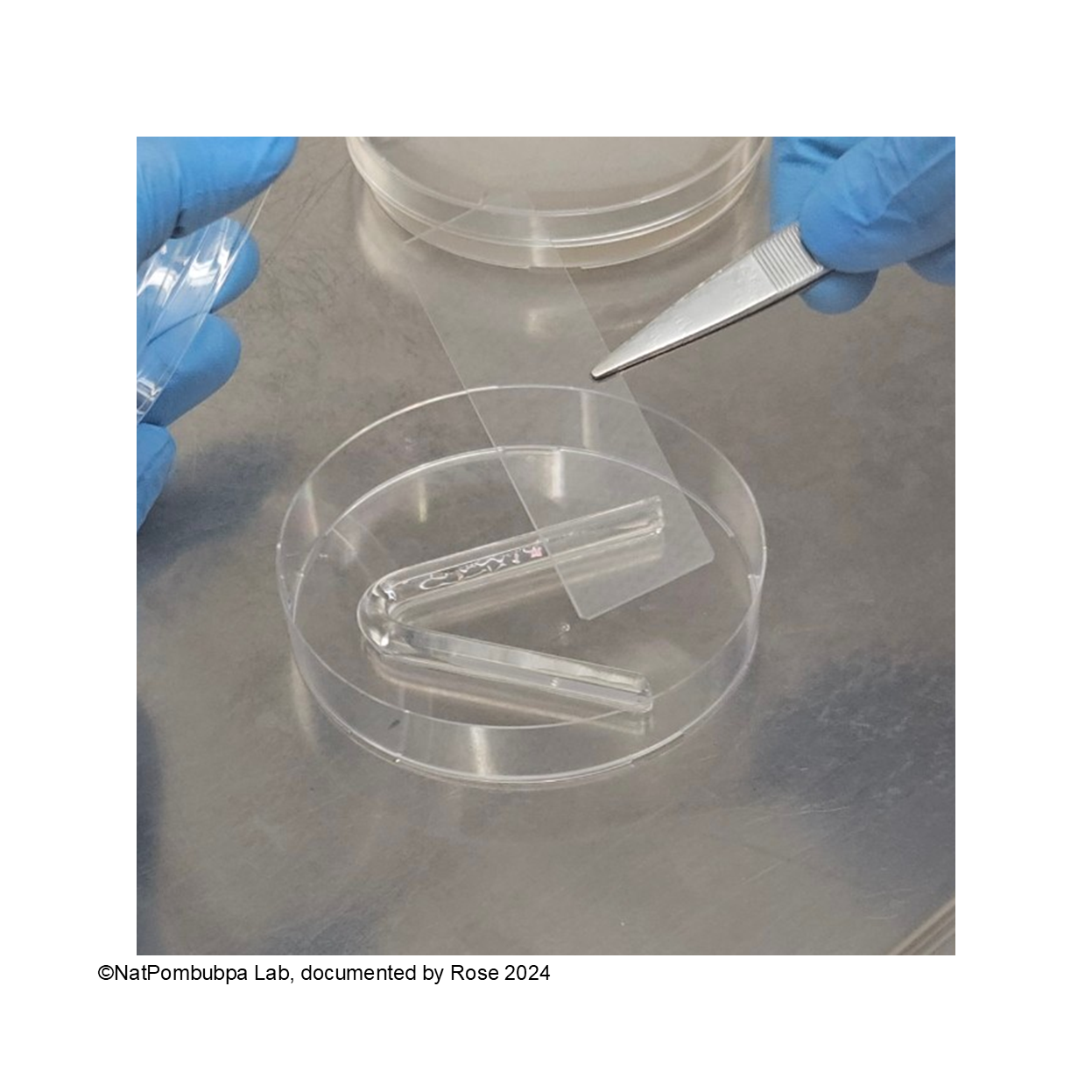
Step 5: Cut the agar to the square shape using sharp side of spatula or scalpel. (Figure 2)
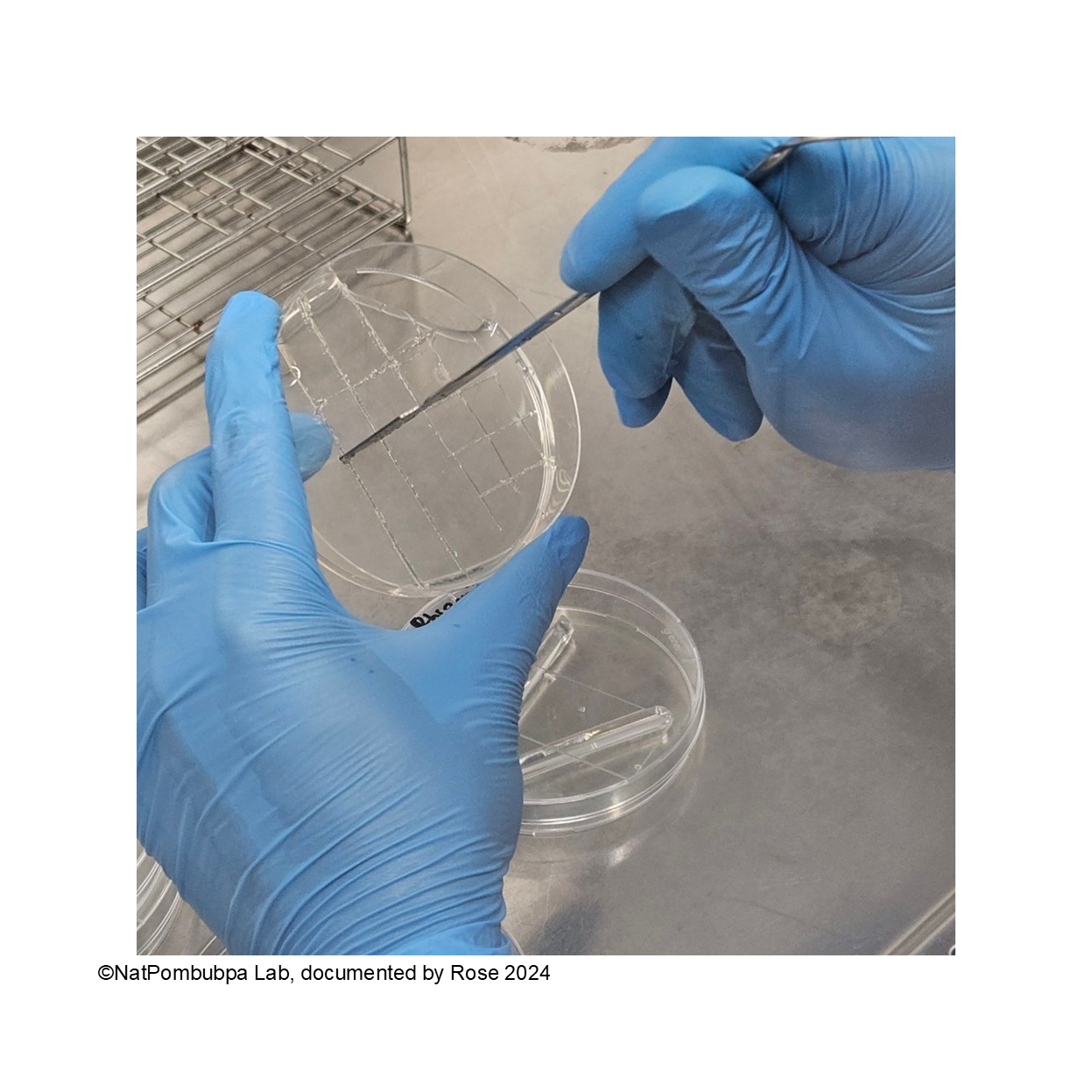
Step 6: Transfer the square shape agar to the slide glass carefully using forceps (Figure 3)
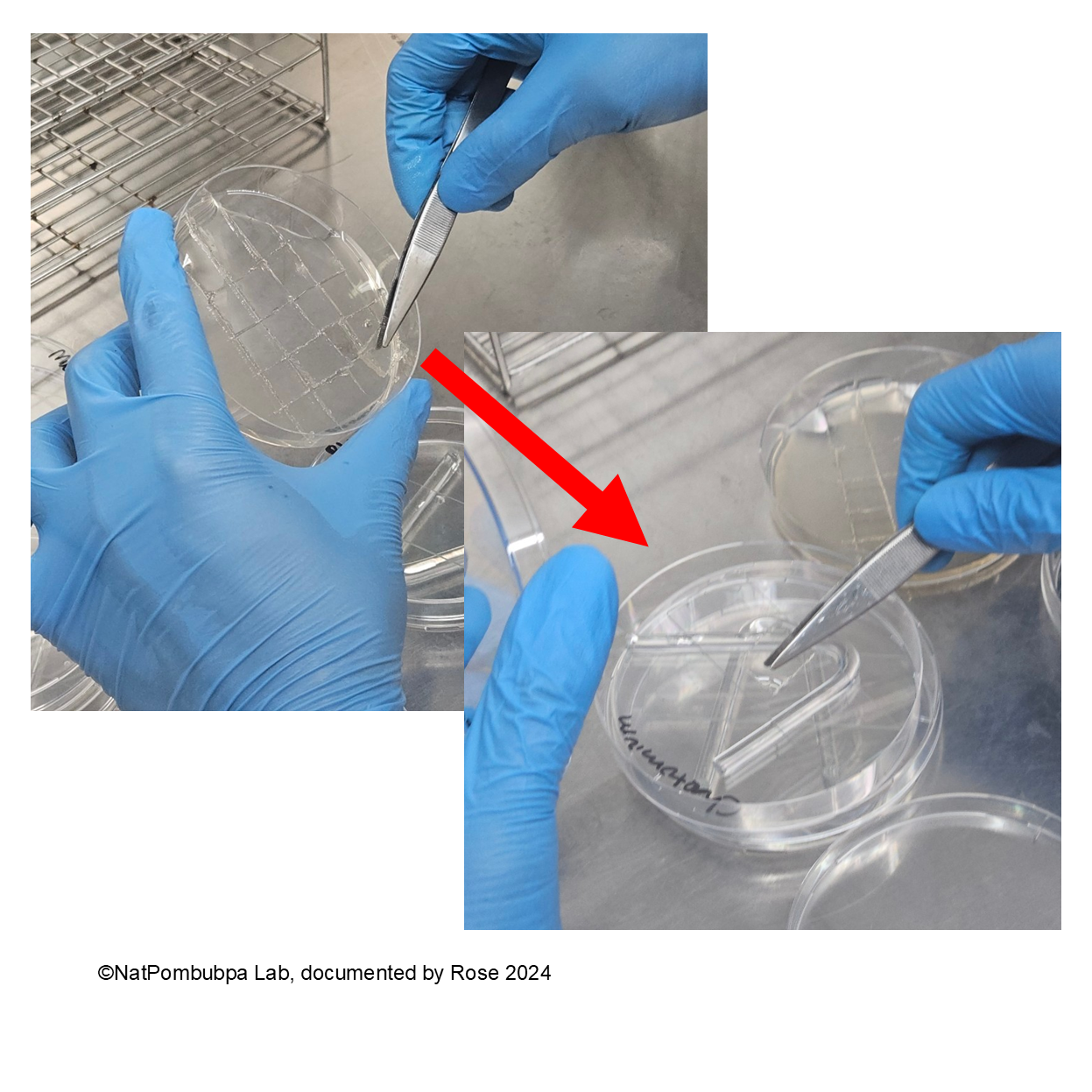
Step 7: Inoculate the fungal from pure colony (5 days age) to the agar using burned needle Ose to get a bit spore. (Figure 4)
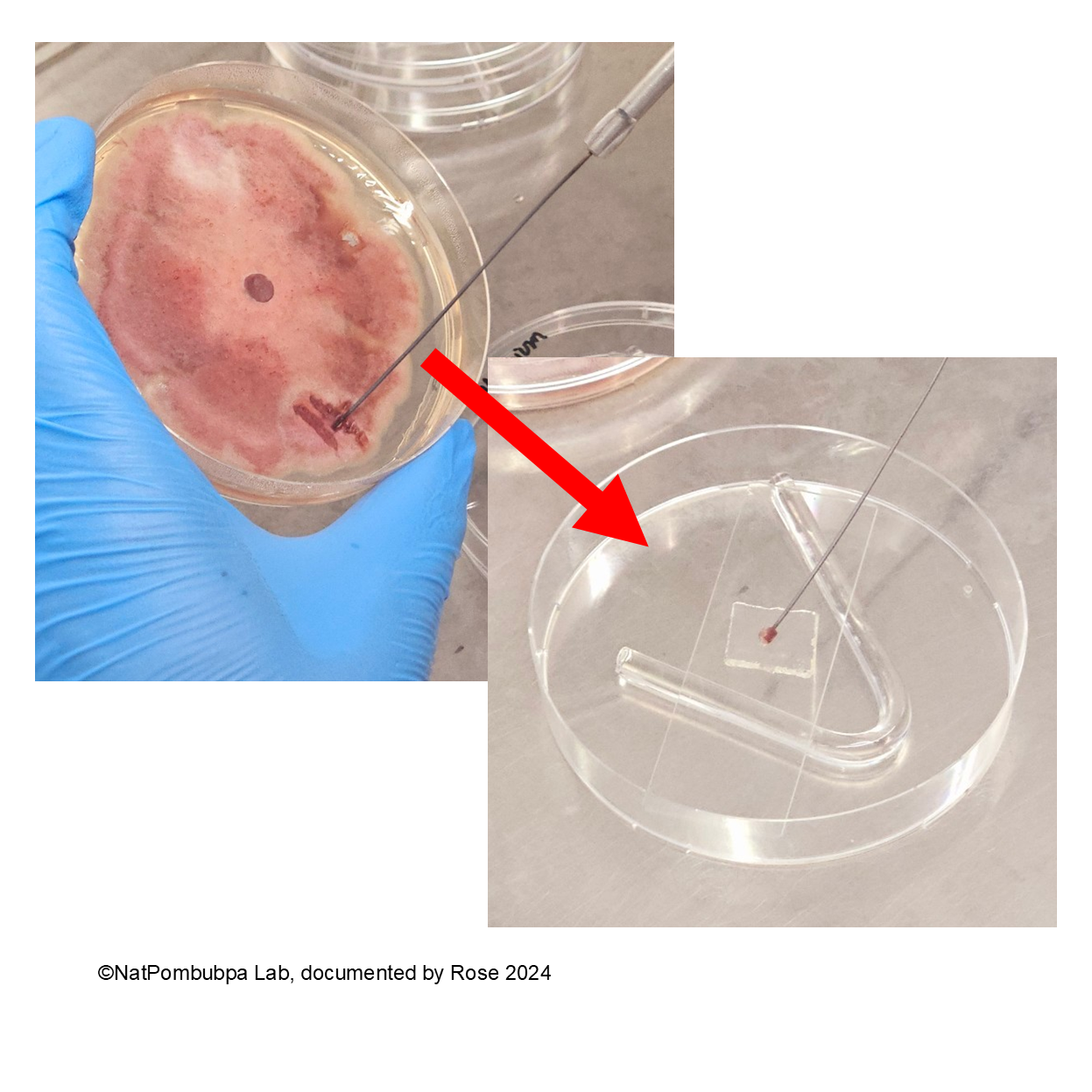
Step 8: Cover the inoculated spore using cover glass and close the Petri dish. Wrap the edge of the dish using parafilm or plastic wrap. Incubate the slide culture for 3 to 5 days. Make sure the spore has already formed based on the colony characteristic before identifying under the microscope. (Figure 5)

The growth fungal colony will be checked under the microscope using 100X or 400X magnification. (Figure 6)

For better resolution of the structure, adding lactophenol blue is recommended. (Figure 7)

References: